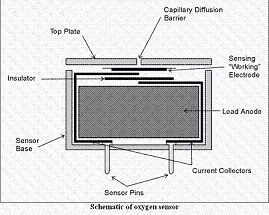
All electrochemical oxygen sensors are of the self-powered, diffusion limited, metal-air battery type comprising an anode, electrolyte and an air cathode as shown.
An oxygen cell can simply be considered as an enclosure (either a metal can or a plastic moulding) which holds two electrodes: a flat PTFE tape coated with an active catalyst, the cathode and a block of lead metal, the anode. This enclosure is airtight apart from a small capillary at the top of the cell which allows oxygen access to the working electrode. The two electrodes are connected, via current collectors, to the pins which protrude externally and allow the sensor to be electronically connected to an instrument. The entire cell is filled with conductive electrolyte which allows transfer of ionic species between the electrodes.